- Home
- Medications
- Erenumab for Migraines
Erenumab for Migraines
~ Guest post by Alycia Gordan. Verified and published by Holly Hazen.
 Erenumab For Migraines. Miracle cure?
Erenumab For Migraines. Miracle cure?Everything you need to know about the new drug that prevents attacks.
Erenumab for migraines was co-developed by two renowned pharmaceutical companies Novartis and Amgen. Erenumab is a human monoclonal antibody that is inoculated to treat migraines. It targets the calcitonin gene-related peptide (CGRP) – a molecule that is responsible for transmitting migraine signals to the brain. [1]
What are migraines?
Migraines are the recurrent headaches which may happen due to specific changes in human brain. They can range from mild to severe. This pain comes with acute sensitivity to sounds, light and/or smell. It brings a profound impact on the ability of individuals in executing daily activities which have led it to become one of the top ten leading causes of years lived with disability for human beings by WHO.
Up to 20% people in United States experience migraines at some point in their lives. With a diversified range of reasons, the exact cause is still not confirmed. MRI scans cannot diagnose a migraine. Nonetheless, the research proves that it occurs due to waves of activity by excitable brain cells. The chemical serotonin narrows down the blood vessels. [3]
Episodic migraines can become chronic. You can read more about the phases of migraine here. Patients falling into the chronic categories are often the ones difficult to treat.
My #1 Choice in Magnesium Supplementation
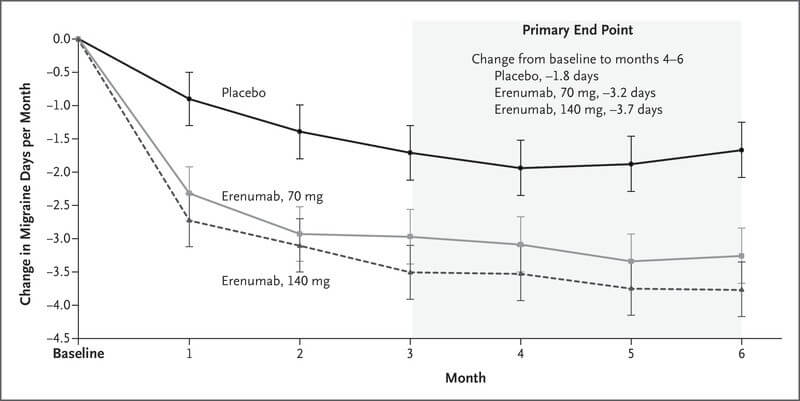
Aimovig (Erenumab) efficacy
Aimovig or Erenumab is the first certified treatment that blocks the CGRP-R. It has resulted in the reduction of monthly migraine days among patients.
According to Sean E. Harper, M.D., executive vice president of Research and Development at Amgen, "The FDA approval of Aimovig represents a long-awaited and important therapeutic development for patients and their physicians who are in need of additional treatment options for the prevention of a migraine." [7]
Liberty III-b
To the efficacy and safety of Erenumab, Novartis conducted a study in 2017/18 which was called Phase III-b LIBERTY. Participants included patients with a history of episodic migraines who had experienced two to four preventive treatment failures. The lack of efficacy or extremity of side effects compelled them to back out with prior plans. [5]
In this study, 246 participants were selected based on the criterion above. They were randomized to receive Aimovig 140 mg or placebo during the 12-week double-blind treatment phase.
The primary result of the study was around 50% reduction in monthly migraine days as compared to the placebo. LIBERTY had some secondary endpoints as well. These included a reduction in days where acute medication was needed, a 75 to 100% positive response days and improved scores on the Migraine Physical Function Dairy Impact tool.
Further study insight
Erenumab for Migraines (Aimovig) has been investigated in various global, random, placebo and double-blind studies to understand its safety and efficacy in prevention of migraines. More than 3000 patients took part in the studies, including LIBERTY as well as an ongoing open-label extension of up to five years in duration.
Erenumab for migraines side-effects
Most reported side-effects of Erenumab include:
- Constipation
- Muscular Spasms
- Itching and Redness
- Pain
- Cramps [2]
The needle cap and shield is made up of dry rubber. It may cause allergic reactions to people who are allergic to latex. [2]
Aimovig may react with other drugs if you intake any. It is integral for the patient to report his/her medical history to their physician. They must provide full details of all the supplements which they use.
As for females, they should tell the doctor if they are pregnant or trying to conceive. There is still no information on how Erenumab can impact upon the fetus. Similarly, there is limited knowledge of Aimovig affecting the breast milk. BF moms should consult their doctor before the injection of Aimovig.
Administration
 Aimovig for Migraine
Aimovig for Migraine Photo credit to Amgen
A visit to a health-care provider is essential. The procedure for Aimovig is that a subcutaneous injection of 70 or 140 mg is given once a month. It is essential to check the single-dose prefilled auto-injector or single-dose prefilled syringe before administration.
The health-care provider shall prescribe any of these two types depending upon the condition of the patient.
If the prescription is 70mg, inject one dose. If the physician prescribes 140mg, two separate injections are needed. In latter case, make use of prefilled auto-injector or prefilled syringe for each injection. The spot for injection has to be different.
Storage of Erenumab
- Store the drug in the refrigerator with temperatures varying between 36°F to 46°F (2°C to 8°C).
- Protecting the drug from light is essential. The original carton would be a good storage option.
- After Aimovig is out of the fridge, it can stay at temperatures varying between 68°F to 77°F (20°C to 25°C) for seven days.
- Freezing it beyond the given temperatures is going to ruin the formula.
- Once the drug crosses the seven day limit at room temperatures, throw it away.
- Do not shake the container before using.
Erenumab For Migraines... Conclusion
The two collaborators, Amgen and Novartis, are devoted to supporting migraine community on a global scale. They plan to help patients by providing them affordable access to Aimovig (Erenumab). They have created the Aimovig Ally™ product support program to aid patients to navigate insurance coverage. As for the ones who are uninsured, they identify potential access resources through this program.
In addition to bringing the new drug to the market, they are also working on changing migraine misperceptions. By educating and changing public perspectives, they are facilitating information among people and providing them the treatment they need.
However, they mention that the public should not place undue reliance on the forward-looking statements used in the press release. Unknown risks and uncertainties, such as the sudden occurrence of mixed dementia may accompany the drug in future. [6]
Despite the disclaimers and side-effects, it won’t be false to state that Aimovig or Erenumab is by far the most effective drug proven to treat a migraine. The dosage may vary according to the patient conditions, and it is essential for the patient to know all possible after-effects.
The future holds high expectations from this medication.
About Alycia Gordan
Alycia Gordan is a freelance writer who loves to read and write articles on healthcare technology, fitness and lifestyle. She is a tech junkie and divides her time between travel and writing. You can find her on Twitter @meetalycia

Stay in touch...
To stay up to date, join the mailing list.
>> Click here to join the mailing list <<
Ready to take the next step?
Choose the next step that fits where you are right now.
MIGRAINE MEDICATIONS Related Articles
Erenumab for Migraines Resources:
1. Durham, P. L. (2006). Calcitonin Gene-Related Peptide (CGRP) and Migraine. Headache, 46(Suppl 1), S3–S8. Available [online] at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3134175/
2. Medscape (2018) Erenumab (Rx). Available [online] at: https://reference.medscape.com/drug/aimovig-erenumab-1000205
3. John Hopkins Medicine (2018) How a Migraine Happens. Available [online] at: https://www.hopkinsmedicine.org/healthlibrary/conditions/nervous_system_disorders/how_a_migraine_happens_85,P00787
4. The New England Journal of Medicine (2018) A Controlled Trial of Erenumab for Episodic Migraine. Available [online] at: https://www.nejm.org/doi/full/10.1056/NEJMoa1705848
5. Novartis (2018) Novartis reports erenumab met all primary and secondary endpoints in unique Phase IIIb study in episodic migraine patients who have failed multiple prior preventive treatments. Available [online] at: https://www.novartis.com/news/media-releases/novartis-reports-erenumab-met-all-primary-and-secondary-endpoints-unique-phase-iiib-study-episodic-migraine-patients-who-have-failed-multiple-prior-preventive
6. BrainTest (2018) Mixed Dementia – Signs & Symptoms. Available [online] at: https://braintest.com/mixed-dementia-signs-symptoms/
7. Harper, S.(2018) Amgen, Executive Vice President, Research and Development. Available [online] at: https://www.amgen.com/about/leadership/senior-management/sean-e-harper/